- Duration: 2018 – 2022
- Funded by the European Union under EDCTP
- Coordinated by Biofabri
- 6 partners
MTBVAC in Newborns: Description and objectives
A new effective tuberculosis (TB) vaccine is essential to achieve World Health Organization End TB goals and eliminate TB by 2050. The optimal long-term strategy would be a combination of serial mass campaigns in adults, coupled with universal newborn vaccination. Newborns are the only human population without prior mycobacterial exposure in TB endemic countries and as such, live attenuated mycobacterial vaccines may offer better protection to this naïve population compared to adults.
MTBVAC is a novel TB vaccine candidate based on an attenuated M. tuberculosis clinical isolate. Safety and immunogenicity of MTBVAC was demonstrated in BCG naïve adults; and MTBVAC appears safe in a Phase 1b study in South African newborns. Definitive demonstration of safety and immunogenicity at the optimal MTBVAC dose is key to progression into multi-centre efficacy trials in infants.
EDCTP supports this Phase 2a dose-defining study of MTBVAC to evaluate the safety, reactogenicity, immunogenicity, and potential for IGRA conversion and reversion, of MTBVAC in South African newborns.
The clinical development consortium will prepare for an infant efficacy trial of MTBVAC by establishing a network of three African sites in South Africa, Senegal and Madagascar. Each site has established research infrastructure. Senegal and Madagascar will, under this project, acquire immunology laboratory technology transfer and training; and collect crucial TB epidemiological data to enable swift transition into a Phase 3 infant trial, pending favourable data from this project.
Aims of the project are:
To evaluate safety and reactogenicity of MTBVAC at three escalating dose levels, compared to BCG vaccine, in healthy, BCG naïve, HIV unexposed, South African newborns;
To evaluate the immunogenicity of MTBVAC at three escalating dose levels in healthy, BCG naïve, HIV unexposed, South African newborns.
A secondary aim of the project is:
To evaluate immunogenicity of MTBVAC at escalating dose levels as compared to BCG
To evaluate the dynamics of MTBVAC vaccine-induced QFT conversion and reversion in healthy, BCG naïve, HIV unexposed, South African newborns.
Project duration 36 months.
Work packages
Work package 1: MTBVAC Phase 2a in newborns
A randomized, controlled, double blind trial will evaluate the safety, reactogenicity, immunogenicity, and potential for QFT conversion and reversion of the candidate TB vaccine MTBVAC in BCG naïve newborns, administered in 3 cohorts at a single intradermal dose of 2.5 x 104, 2.5 x 105 or 2.5 x 106 CFU, compared to BCG vaccine at a dose of 2.5 x 105 CFU.
The hypothesis is, that the optimal safe and immunogenic MTBVAC dose for infants lies in the range of 2.5 x 104 (10-fold lower than BCG) to 2.5 x 106 CFU (10-fold higher than BCG). Therefore, this proposed infant trial aims to define the MTBVAC dose-response curve above and below the optimal MTBVAC dose, straddling a 3-log dose range.
In addition, given the crucial importance of confirming that MTBVAC vaccine-induced QFT conversion occurs in infants, understanding the immunological basis for MTBVAC vaccine-induced QFT conversion, and defining the time course and magnitude (IFN-g concentration) of MTBVAC vaccine-induced QFT conversion and reversion events, serial QFT assays will be performed in parallel with whole blood T cell assays.
Study Design: Ninety-nine HIV unexposed, BCG naïve, newborns without known household exposure to M.tuberculosis will be randomized to receive either BCG standard dose at 2.5 x 105 CFU (n=24) or MTBVAC at one of three different dose levels (n=75).
The work package is subdivided into three sub-work packages:
- Study start-up
- Study conduct
- Study close out
Work package 2: Capacity building and Technology Transfer for Infant Efficacy Trials
The clinical development consortium will prepare for the planned MTBVAC newborn efficacy trials by establishing a network of three sites in three African countries; i.e. South Africa: South African Tuberculosis Vaccine Initiative (SATVI) at the University of Cape Town (UCT); Senegal: Centre de Recherche Biomédicale (CRB-EPLS) and Madagascar: Institut Pasteur de Madagascar (IPM). These three sites, which include Francophone and Anglophone institutions in Southern, East and West Africa, have been selected to offer optimal genetic, epidemiologic, and demographic diversity in the study population for future efficacy testing. Each site has an established research infrastructure. In order to establish readiness for clinical trials of TB vaccines, the Senegal and Madagascar sites will, under this project, further develop immunology laboratory capacity through technology transfer and training.
The primary capacity building goals include:
- To standardize immunological assay techniques;
- To harmonize common operational practices and standard operating procedures.
Capacity building will be ensured through several phases:
- Training phase by SATVI for technicians from CRB-EPLS and IPM
- Local transposition and translation of Standard Operating Procedures
- Technology transfer phase
- Validation phase
Work package 3: Site Preparation Study: Epidemiology of Childhood TB in partner sites
A cross–sectional childhood TB prevalence survey will be conducted in Senegal and Madagascar to collect information on the local TB epidemic to inform site selection, sample size, and recruitment strategies for a future efficacy trial of MTBVAC in young children. These studies are focused on estimating likely accrual of childhood TB disease endpoints in targeted study communities.
Specifically, these studies will provide targeted prevalence estimates among high-risk sub-communities who would be identified for recruitment in an efficacy trial. This WP will build upon the enhanced laboratory capacities acquired in WP2 to conduct a cross-sectional survey of age-specific M. tuberculosis infection rates during which additional epidemiological data will be gathered on TB disease prevalence.
The objectives are to provide epidemiological data on childhood TB disease prevalence to prepare for future efficacy trials with MTBVAC at the Senegal and Madagascar study sites:
- Define local age-specific prevalence rates of TB infection and disease in children by respectively QuantiFERON® assay and available data from the local, regional, or national TB Control Program;
- Establish the respective endpoints of future Prevention of Infection (POI) and Prevention of Disease (POD) efficacy trials;
- Infer burden of TB transmission and local childhood TB incidence.
This work package is subdivided into three sub-work packages:
- Establishment of the protocol and approvals
- Cross-sectional childhood TB prevalence survey
- Data management and analysis
Work package 4: Project management
To ensure and manage the expected project objectives, a project management structure is established in order to:
- Establish and implement management infrastructure (Steering Committee, Project management team, Scientific and Clinical Advisory Team, Project management tools (administration, financial, dissemination and communication);
- Scientific and technical coordination of the project activities;
Financial and contractual management; - Monitor project progress, and provide reports;
Monitor data management and impact; - Implement dissemination and communication activities;
- Monitor ethical issues.
This work package will be distributed into two sub-work packages:
- Establish organizational structure including its bodies
- Implementation of the coordination and project management activities
EDCTP
MTBVAC is a novel TB vaccine candidate based on an attenuated M. tuberculosis clinical isolate. Safety and immunogenicity of MTBVAC was demonstrated in BCG naïve adults; and MTBVAC appears safe in a Phase 1b study in South African newborns. Definitive demonstration of safety and immunogenicity at the optimal MTBVAC dose is key to progression into multi-centre efficacy trials in infants.
EDCTP supports this Phase 2a dose-defining study of MTBVAC to evaluate the safety, reactogenicity, immunogenicity, and potential for IGRA conversion and reversion, of MTBVAC in South African newborns.

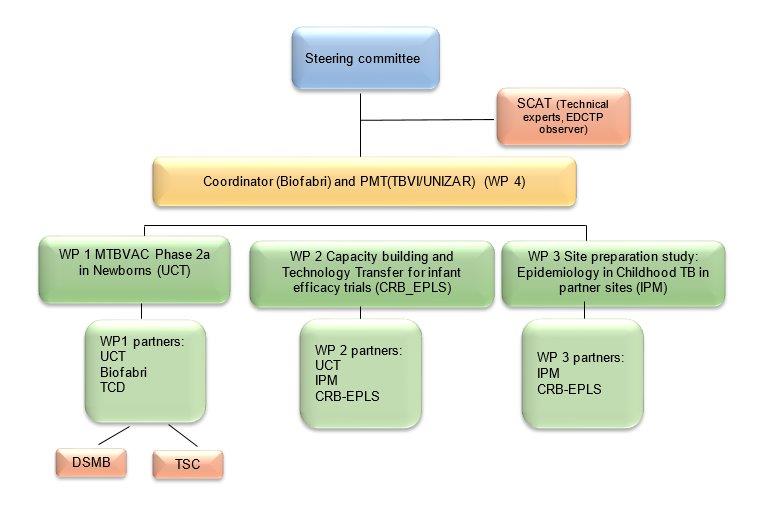
Governance Structure
The MTBVAC-NEWBORN consortium is composed of 3 European and 3 sub-Saharan African participants. Two participants are Universities (UCT, UNIZAR), two are Higher Education Institutions (CRB-EPLS, IPM), one is a product development partnership, supporting and coordinating TB vaccine development (TBVI), and one is private industry (BIOFABRI).
Coordinator & Partners

Biofabri focuses on two strategic lines of business: on the one hand, the development and biological production of vaccines and, on the other, the manufacturing and distribution of biopharmaceutical specialties and biotech services for third parties.
Amongst the most advanced projects in its pipeline is the promising vaccine candidate against Tuberculosis, MTBVAC.
The MTBVAC vaccine, developed and manufactured entirely in Spain, is one of the most promising vaccines against tuberculosis in the portfolio of vaccines candidates. MTBVAC is a live attenuated freeze-dried vaccine developed by Biofabri, from a strain designed by the research group of Carlos Martin of the University of Zaragoza (Spain) next to the Pasteur Institute
Biofabri as Coordinator within the Global Consortium will act as the intermediary between the beneficiaries and the EDCTP Association and shall perform all tasks assigned to it as described in the Grant Agreement.
The Consortium partners

University of Zaragoza (Unizar): As the discoverer and initial developer of MTBVAC, Unizar works in close collaboration with industrial partner Biofabri providing longstanding preclinical, immunological and scientific expertise on the molecular bases of attenuation and protection of MTBVAC. Unizar will provide advise on the proposed immunological, clinical and capacity building activities and scientific support to the project management activities. Unizar will also provide scientific advice to all partners on the Clinical Trial Protocols and corresponding immunological studies and will contribute to the immunological validation of once-identified TB-diagnostic test suitable for MTBVAC clinical evaluation and the coordination of immunological studies.

TBVI provides its longstanding project management and coordination expertise to support the coordinator (Biofabri) in its role to coordinate and manage the MTBVAC Phase 2a project. In addition, TBVI provides its regulatory and clinical expertise to advise on the immunological, clinical and capacity-building activities.

SATVI/UCT SATVI is a university-accredited TB research centre established in 2001 and located within the University of Cape Town (UCT), South Africa. It is the largest dedicated TB vaccine research group on the African continent. SATVI’s research scope spans several disciplines including paediatrics, infectious diseases, epidemiology, public health, immunology, systems biology and clinical sciences. As partner to the Phase 2a trial, SATVI will utilize its extensive experience to carry out the clinical trial and capacity building activities of the grant.

CRB-EPLS in Senegal provides their extensive local, clinical and immunological expertise to MTBVAC safety and immunological evaluation in newborns and research of potential biomarkers of vaccine-induced protection.

TCD / FHI Clinical SA: Triclinium Clinical Development (TCD) is a Clinical Research Organization (CRO) headquartered in Centurion (Pretoria), South Africa, providing end-to-end clinical development services. Recently TCD became part of FHI Clinical. The new organisation, called FHI Clinical SA will provide both operational services such as regulatory, regional project management, clinical monitoring & site management, medical monitoring & pharmacovigilance as well as back-end services such as data management, biostatistics and medical writing to support clinical research activities across the Sub-Saharan region. For on-site monitoring activities, FHI-Clinical SA will deploy its personnel located strategically across Sub-Saharan region.
Publications & News
April 2025
Publication Safety, reactogenicity, and immunogenicity of MTBVAC in infants: a phase 2a randomised, double-blind, dose-defining trial in a TB endemic setting Publication by Michele Tameris, et al. on behalf of the MTBVAC 202 study team, DOI 10.1016/j.ebiom.2025.105628
December 2021
Publication MTBVAC, a live TB vaccine poised to initiate efficacy trials 100 years after BCG
Publication by Carlos Martín, Dessislava Marinova, Nacho Aguiló, Jesús Gonzalo-Asensio;
DOI: 10.1016/j.vaccine.2021.06.049
7 June 2021
Third annual project meeting MTBVAC in new-borns Phase 2a
The consortium of ‘MTBVAC in newborns Phase 2a’ held its third annual project meeting virtually on 31 May to discuss project progress.
The meeting, led by the coordinator Biofabri, was attended by all partners including the Scientific and Clinical Advisory Team (SCAT). The SCAT and Biofabri presented the clinical development plan and next Phase 3 trial, and the newest insights of pre-clinical assays of MTBVAC and exploratory immunogenicity endpoints.
This Phase 2a study aims to evaluate safety, reactogenicity and immunogenicity of MTBVAC at three escalating dose levels, compared to BCG vaccine, in healthy, BCG naïve, HIV unexposed, South African newborns.
Despite of the Covid-19 pandemic effects, two years after the first vaccination, the last vaccination was given in March 2021. In total 99 healthy, newborns are enrolled and followed for 12 months, therefore, a final analysis can be made in April 2022.
To move straight from the Phase IIa to Phase III study (RIA2019S-2652), a DSMB review in a blinded manner to determine and assess the most relevant immunogenic and safe MTBVAC dose will take place at D182 of the last vaccination, therefore, September 2021
The design of the proposed Phase III incorporates three case-driven assessments for futility and efficacy. Such a pathway would allow for earlier availability of the neonatal vaccine. Such a saving in time would have a major public health benefit when considering that the BCG vaccine has been reported to have variable protective effect.
18 March 2021
MTBVAC in new-borns Phase 2a trial completed its enrolment
Two years after the first new-born baby for the MTBVAC Phase 2a trial was enrolled, the enrolment of the study has completed. Early March the last baby in the trial received a vaccination.
The South African Tuberculosis Vaccine Initiative (SATVI) conducted the study in the Western Cape of South Africa, recruiting women in the third trimester of pregnancy, following them through delivery before enrolling their newborns. Babies are followed up over a period of 12 months. In total 99 healthy, BCG naïve, HIV unexposed newborns are enrolled into this study.
The completing of the enrolment is specially celebrated due to a challenging 2020 with unexpected and unavoidable major issues, namely the COVID-19 pandemic and lockdown in South Africa.
The restrictions imposed by the COVID-19 lockdown in South Africa impacted the duration of the study. These lockdown measures were implemented, firstly, to prevent rapid spread of infection and, secondly, to assist government in preparing for a potential increase in COVID-19 admissions to hospitals causing strain on the South African health care system. Therefore, measures resulted in closure of all sectors whilst only allowing essential services to proceed until beginning October 2020.
Fortunately, the study activities resumed from October 2020, which resulted in the completion of the enrollment early March.
This trial is funded and supported by the European and Developing Countries Clinical Trials Partnership (EDCTP).

The first new-born baby that was enrolled for the Phase 2a study in 2019 and the last baby that was enrolled for this study in 2021 with their mothers and the Satvi-team.
A Phase 3 efficacy study is planned to start for early 2022. This will be an important step with the planned MTBVAC vaccination of nearly 7 000 infants. This large, phase 3 clinical trial is partially funded and supported by EDCTP.
MTBVAC in new-borns Phase 2a trial completed its enrolment – Zendal
7 June 2021
Third annual project meeting MTBVAC in new-borns Phase 2a
The consortium of ‘MTBVAC in newborns Phase 2a’ held its third annual project meeting virtually on 31 May to discuss project progress.
The meeting, led by the coordinator Biofabri, was attended by all partners including the Scientific and Clinical Advisory Team (SCAT). The SCAT and Biofabri presented the clinical development plan and next Phase 3 trial, and the newest insights of pre-clinical assays of MTBVAC and exploratory immunogenicity endpoints.
This Phase 2a study aims to evaluate safety, reactogenicity and immunogenicity of MTBVAC at three escalating dose levels, compared to BCG vaccine, in healthy, BCG naïve, HIV unexposed, South African newborns.
Despite of the Covid-19 pandemic effects, two years after the first vaccination, the last vaccination was given in March 2021. In total 99 healthy, newborns are enrolled and followed for 12 months, therefore, a final analysis can be made in April 2022.
To move straight from the Phase IIa to Phase III study (RIA2019S-2652), a DSMB review in a blinded manner to determine and assess the most relevant immunogenic and safe MTBVAC dose will take place at D182 of the last vaccination, therefore, September 2021
The design of the proposed Phase III incorporates three case-driven assessments for futility and efficacy. Such a pathway would allow for earlier availability of the neonatal vaccine. Such a saving in time would have a major public health benefit when considering that the BCG vaccine has been reported to have variable protective effect.
30 May 2020
Virtual project meeting ‘MTBVAC in newborns’
Originally the intention was to have a site visit at IPM in Madagascar this year. Due to the Covid-19 pandemic, a site visit was not possible so the project meeting was held virtually on May 28. All partners of the project attended including the members of the SCAT (the Scientific and Clinical Advisory Team).
Each partner presented an update about the progress and the SCAT presented the next steps in the clinical development plan. Also, the impact of the Covid-19 pandemic on the project was discussed and it became clear that this will cause some uncertainties in the project. Despite this uncertainty, progress has been made and some work packages have almost completed.
Depending on how the Covid-19 pandemic develops, the site visit to IPM in Madagascar is postponed to next year.
March 2021
Publication BCG vaccination improves DTaP immune responses in mice and is associated with lower pertussis incidence in ecological epidemiological studies
Esther Broset, Jacobo Pardo-Seco, Alex I Kanno, Nacho Aguilo, Ana Isabel Dacosta, Irene Rivero-Calle, Jesus Gonzalo-Asensio, Camille Locht, Luciana C C Leite, Carlos Martin, Federico Martinón-Torres DOI: 10.1016/j.ebiom.2021.103254
April 2020
Publication Update on TB Vaccine Pipeline
Publication by Carlos Martin, Nacho Aguilo, Dessislava Marinova and Jesus Gonzalo-Asensio; https://doi.org/10.3390/app10072632
9 March 2020
Partners ‘MTBVAC in newborns’ participate in TBVI meeting and a joint meeting from EDCTP funded TB-projects
All partners of the project attended the TBVI meeting “Accelerating the TB vaccine pipeline – towards Horizon Europe” from 27-29 January 2020. This meeting brought together 120 scientists and policy makers from 70 research institutes, universities, industry, funding agencies, technical agencies from 21 countries.
In parallel to this meeting a joint meeting was organised with EDCTP funded TB-projects. EDCTP is funding three TB vaccine clinical trial projects knowing POR TB (coordinated by Statens Serum Institute, Denmark), priMe (coordinated by Vakzine Projekt Management GmbH, Germany) and MTBVAC in newborns (coordinated by BioFabri, Spain).
To enhance the collaboration between the projects and to maximise the impact, TBVI, commissioned by EDCTP, will develop tools that may support these joint project activities and help future TB vaccine clinical trial projects. This meeting in Les Diablerets was a kick off and meant to explain the purpose of the tools and the joint activities that will be developed.
EPLS as well as IPM presented their clinical sites during this meeting:

25 March 2019
World TB Day: EDCTP investments in TB research rise to EUR 127 million
In 2018, three major grants were committed to support the development of several candidate TB vaccines: a total of EUR 31.5 million for three large clinical trials. Currently, these clinical trials conducted by 28 research institutions from sub-Saharan Africa, Europe and India, are well underway. The projects are PORTB, priME and MTBVAC in newborns. Please read the article on the EDCTP website.
18 February 2019
First enrolment into MTBVAC 202 – a Phase 2a trial in newborns
Today the first newborn baby for the MTBVAC 202 Phase 2a trial of a novel TB vaccine was enrolled onto the study and received her study vaccination. This trial is being conducted in the Western Cape, South Africa by the South African Tuberculosis Vaccine Initiative (SATVI), University of Cape Town.
In total 99 healthy, BCG naïve, HIV unexposed newborns will be enrolled onto the study in the coming months and carefully followed up over a 12 month period.
According to the project coordinator Dr Ingrid Murillo this study marks an important step forward in the development of MTBVAC. This trial, funded and supported by European and Developing Countries Clinical Trials Partnership (EDCTP) will build the bridge to the efficacy trial, the next MTBVAC challenge.


14 February 2019
Publication “Vaccination against tuberculosis”
The article ‘Vaccination against tuberculosis’ written by C Martin, N Aguilo and J Gonzalo-Asensio Enferm has been published: Infecc Microbiol Clin. 2018 Dec;36(10):648-656. Doi 10.1016/j.eimc.2018.02.006.
14 December 2018
“Dose-Defining Safety and Immunogenicity Study of MTBVAC in South African Neonates”
For information about the clinical trial, please follow the link “Dose-Defining Safety and Immunogenicity Study of MTBVAC in South African Neonates” (ClinicalTrials.gov: NCT03536117).
25 September 2018
Joint symposium at the EDCTP Forum 19 September 2018: ‘TB vaccine development: the results of unique collaborative effort’
At the EDCTP Forum that was held from 17-21 September 2018 in Lisbon, four EDCTP projects on TB vaccine clinical development presented their vaccine candidate during a symposium ‘TB vaccine development: the results of unique collaborative effort’.
The symposium provided an overview of recent progress in the development of TB vaccines and the results from collaborative efforts. It also highlighted the crucial role that EDCTP is playing in the clinical development of TB vaccine candidates.
The introductory presentation covered the current state of the TB vaccine pipeline and described a stage gating approach developed by Aeras and TBVI. The developed webtool ‘TB Vaccine Development Pathway’ for the TB vaccine community supports the development of novel vaccine candidates.

This presentation was followed by a series of presentations on four large TB vaccine clinical trials recently funded by EDCTP. 28 Research institutions from sub-Saharan Africa, Europe and India will work closely together on these trials.
These trials will evaluate the three different vaccine candidates (H56:IC31, VPM1002 and MTBVAC) and represent the largest European investment into clinical development of TB vaccines to date. Finally Aeras presented a collaborative approach to community engagement.
19 June 2018
Kick Off Meeting 6 and 7 June 2018 in Cape Town, South Africa
On 6-7 June the kick off meeting of MTBVAC in Newborns took place in Cape Town, South Africa. This project contains a Phase 2a dose-defining safety and immunogenicity study is being carried out. The project includes also capacity building to support vaccine efficacy trials in Tuberculosis-endemic regions of Sub-Saharan Africa. The project is part of the EDCTP2 programme supported by the European Union.
Project partners from South Africa (Satvi, UCT), Senegal (CRB-EPLS), Madagascar (IPM), Spain (Biofabri and Unizar) and the Netherlands (TBVI) attended the meeting. Also a representative of EDCTP, Tom Nyirenda, joined the kick off of this project. Furthermore, the Steering Committee held their first face-to-face meeting.
Work package leaders and members had the opportunity to be introduced to each other and to discuss about the study approach. The South African partner Satvi hosted also a visit to their field site. The meeting turned out to be a wonderful and promising start of the project.


Communication Tools
Scientific Publications:
EDCTP recommends the following statement to acknowledge EDCTP funding in online or printed articles:
“This project is part of the EDCTP2 programme supported by the European Union (grant number RIA2016V-1637-MTBVAC in Newborns)”
Promotional materials:
EDCTP recommends the following statement to acknowledge EDCTP funding in publication: “This publication was produced by MTBVAC in Newborns which is part of the EDCTP2 programme supported by the European Union (grant number RIA2016V1637-MTBVAC in Newborns). The views and opinions of authors expressed herein do not necessarily state or reflect those of EDCTP.”
Presentations:
Use the logo below of EDCTP and EC for your presentations:

