The philosophy and spirit of TBVI and its consortium is one of true partnership and collaborative research efforts. The TBVI R&D strategic directions and priorities are based on the scientific and strategic inputs of its consortium members, its Advisory Committee, its P&CDT experts and other partners. This active, ongoing dialogue enables TBVI to stay at the cutting edge of TB vaccine and biomarker R&D.
A continuous dialogue with consortium partners
TBVI encourages and enables scientists and researchers to bring forward their innovative ideas, concepts, technologies and strategies for a creative ‘bottom up’ approach to vaccine and biomarker discovery and development.
TBVI does not have its own commercial interests. Ownership of vaccine candidates and biomarkers and intellectual property rights stay with researchers and vaccine developers. TBVI supports individual research organisations and developers to manage IP issues in accordance with their strategic interests and the need to provide affordable and accessible products for the end user.
Active networking for knowledge generation and exchange
TBVI provides project management and oversight but more importantly, uses its best practices and platforms to facilitate knowledge exchange, promote synergies and supports collaborative research.
TBVI brings R&D organisations, scientists and industry partners together in one network through organising scientific meetings where knowledge sharing is promoted and incentivised. It links research organisations and universities to SMEs and the vaccine industry in order to facilitate optimal development of vaccine candidates in clinical settings.
Transparency and confidentiality
Transparency underpins the work of TBVI. The Articles of Association TBVI, policies and rules of procedure, audited financial statements and governance-related documentation are available on request.
Safeguards are in place to manage possible conflicts of interest or any perception of conflicts of interest. Any professional working with TBVI is asked to declare her/his conflicts of interest related to TBVI or the vaccine candidates. Similarly, external experts who advise TBVI are required to comply with TBVI’s conflict of interest policy.
TBVI treats data received from its collaborators with strict confidentiality.
TBVI requires that all clinical trials funded by TBVI or in which TBVI experts are advisers are to be performed in accordance with the standards and codes of conduct accepted by the International Conference on Harmonisation (ICH) guidelines and in compliance with the local ethical and regulatory requirements.
The process of calling for project proposals
Depending on the nature of a project or the conditions of funding, TBVI calls for proposals from its network partners for the funding of specific activities by the following modalities:
- open call: a call for any relevant type of activity without limitation on the number of applicants
- a targeted call: a call addressing two or more pre-identified partners that will be invited to respond in the context of a specific activity
- dedicated individual call: in case of a project opportunity or activity that is highly specific and well-defined, TBVI may call upon a network partner that is best capable of delivering on the specific activity
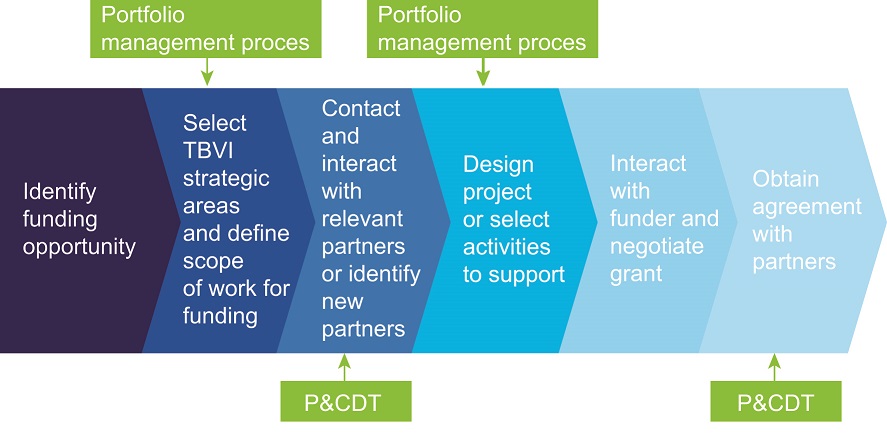
Project identification, design and development in steps
TBVI has a transparent process in place to connect new funding opportunities to R&D priorities in the field. Decisions on the design of a project and allocation of funding are based on close interaction with relevant partners and on the bottom-up process of calling for proposals from partners in the network. The following steps are applied:
- TBVI identifies funds for TB vaccine, biomarker R&D projects
- R&D areas are selected on the basis of appropriateness of funding conditions and complementarity with TBVI strategies and identified R&D priorities. The Portfolio Management Committee (PMC) provides advice and guidance in this process
- network partners are invited to apply via a call for proposals (see above)
- the PMC reviews the applications, prepares a decision matrix based pre-established criteria, and advises on prioritisation for funding
- the TBVI Executive Director makes the final decision
- TBVI, together with the selected partners, elaborates the specific R&D content of the project. If needed, advice is sought from the PMC or specific P&CDT members
- when applicable an ethical review of the approved proposal is carried out by independent reviewers
- TBVI interacts closely with the funders to ensure the project meets the aims and prerequisites of their funding conditions
- a grant agreement with each partner is negotiated. In principle, TBVI expects partners to provide for co-funding when they are selected for funding through TBVI
- P&CDT proactively manages and supports the progress of activities of the project partners
Figure : TBVI’s funding and prioritisation process
The TBVAC2020 example
- TBVI identified the European Commission Horizon 2020 call on new TB vaccines with a maximum available budget of €25 million
- TBVI, in consultation with leading experts in the field, identified the following areas and scope of work to be included in the project proposal: discovery, preclinical, biomarkers, early clinical development and portfolio management
- to enable the involvement of new partners and to receive the best new ideas, TBVI launched an open call for Expression of Interest (EoI)
- the call was published on the TBVI website and sent out to all 2,500 contacts and newsletter subscribers all over the world. Over 100 EoI letters were received
- a project selection committee – comprised of a selection of TBVI’s P&CDT, TBVI staff and experts – selected the best project proposals based on pre-determined selection criteria. A total of 54 proposals were selected and included in the grant proposal that was submitted to the EC Horizon 2020 programme
- the EC selected TBVI’s project for further negotiation and offered a contribution of €18.2 million for the project
- a round of consultation with the anticipated project partners resulted in a final proposal and agreement with the EC for a budget of €18.2 million. Additional funds from the Swiss, Korean and Australian governments complemented the project budget for a total of €24.6 million
Check our current partners and read how to become a partner.