Novel immunotherapies for tuberculosis and other mycobacterial diseases
Funded by:


Project Overview
To establish a critical path to select promising innovative adjunct TB immunotherapies and to use this to progress 2 immunotherapies to completion of preclinical proof of concept in NHPs
- Duration: June 2023 – May 2027
- Funded by the European Union under HaDEA; type of action HORIZON-RIA
- Coordinated by TBVI
- 11 partners
Aims and objectives
Tuberculosis (TB) remains the leading cause of death due to a single pathogen, Mycobacterium tuberculosis (Mtb), with 1.3 million deaths in 2022. The lengthy TB treatment and the numerous adverse events contribute to poor medical adherence and development of antibiotic resistant strains. Thus, novel therapeutic modalities are urgently needed to shorten treatment duration, improve outcomes and control the emergence of drug resistant TB.
The ITHEMYC project convenes a multidisciplinary consortium of 11 partners, including two Product Development Partnerships (TBVI, TB-Alliance) and an industrial partner (GSK) involved in vaccine, drug and biomarker R&D for TB. The partners will work jointly to develop innovative adjunctive TB immunotherapies by capitalizing on a promising pipeline and recent developments in the field. The project will combine current and new antibiotic regimens with novel immunotherapies, such as small molecules targeting host pathogen-interactions, including host-directed therapies and virulence inhibitors, immunomodulatory compounds, monoclonal antibodies and therapeutic vaccines. The project will generate robust preclinical safety and efficacy information on compounds and combinations through a set of relevant in vitro, in vivo and in silico models, and progress two of them up to preclinical proof-of-concept in non-human primates within the project duration. The partners are proposing a critical path for characterization and progression of immunotherapies, that will be refined based on knowledge generated in ITHEMYC aiming to increase the understanding and interest for this emerging concept of adjunctive TB immunotherapy. We expect the new combined interventions will improve TB cure rates, reduce the duration and toxicity of current regimens and reduce relapse rates.
Work packages
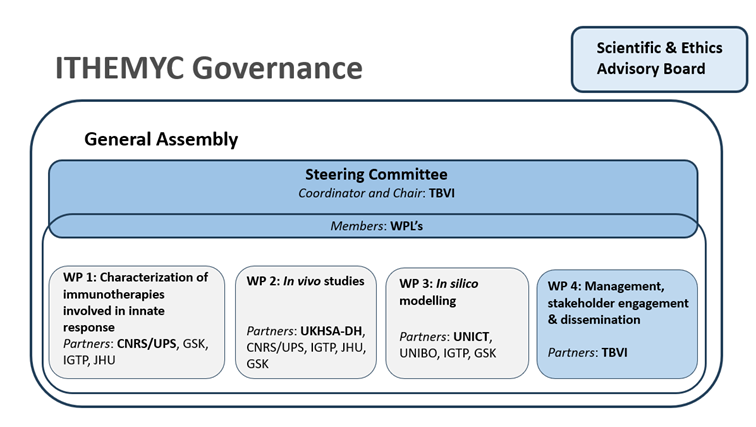
WP1: Characterization of immunotherapies involved in innate response
WP2: In vivo studies
WP3: In silico modelling
WP4: Management, stakeholder engagement and dissemination & exploitation
WP5: Ethics requirements
Project governance
The ITHEMYC management structure was designed to optimally support this project and consists of a General Assembly (GA), WP Leaders (WPL), a Steering Committee (SC) and a Management Team (MT). The consortium will be advised by a Scientific and Ethics Advisory Board (SEAB).
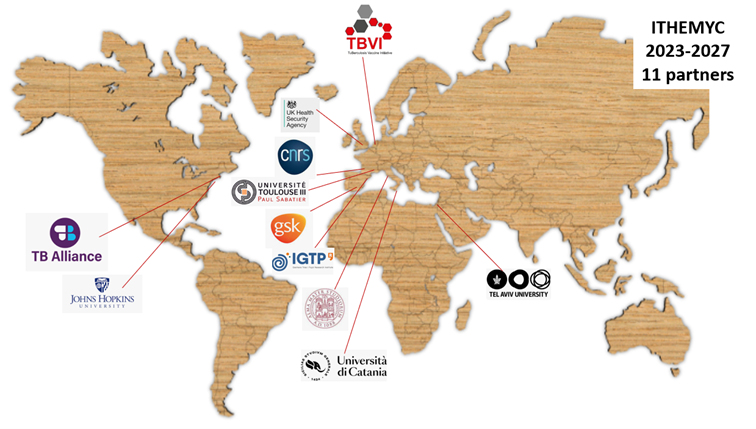
Consortium Partners
The ITHEMYC consortium consists of 11 partners, bringing together the different kinds of expertise needed for this multidisciplinary project and located in seven different countries.
Publications
Lung organoids as a human system for Mycobacteria infection modeling and drug testing
Stephen Adonai Leon-Icaza, Romain Vergé, Raoul Mazars, Laurence Berry, Céline Cougoule
FEBS Journal, https://doi.org/10.1111/febs.70265
Published: 21 September 2025
Drosophila melanogaster experimental model to test new antimicrobials: a methodological approach
Maria Vidal, Marta Arch, Esther Fuentes, Pere-Joan Cardona
Frontiers in Microbiology, 10.3389/fmicb.2024.1478263/full
Published: 6 November 2024
News
February 2024 – ITHEMYC meeting in Les Diablerets
On January 29-30, 2024, the ITHEMYC consortium held its Annual meeting in Les Diablerets, Switzerland. The aim of this meeting was to update, discuss and advance the project in a face-to-face meeting with all consortium partners.
June 2023 – ITHEMYC kicks off
Novel Immunotherapies for Tuberculosis and other Mycobacterial Diseases (ITHEMYC)
The Horizon Europe programme has funded the ITHEMYC project to establish a critical path for selection of promising innovative adjunct TB immunotherapies and progress 2 of these immunotherapies to completion of preclinical proof of concept.

