The project “MTBVACN3” aims to perform a Randomised, Double-blind Controlled Phase 3 Trial to evaluate the efficacy, safety and immunogenicity of MTBVAC-vaccine administered in healthy HIV unexposed uninfected, and HIV exposed uninfected newborns in Tuberculosis-endemic regions of Sub-Saharan Africa.
A new effective TB vaccine is essential to achieve World Health Organization End TB goals and eliminate TB by 2050. The optimal long-term strategy would be a combination of serial mass campaigns in adults, coupled with universal newborn vaccination. Newborns are the only human population without prior mycobacterial exposure in TB endemic countries. This project is based on the hypothesis that live attenuated mycobacterial vaccines will offer better protection to this naïve population compared to adults.


The objective of this project is to demonstrate safety, immunogenicity and improved efficacy of the new live attenuated M.tuberculosis vaccine called MTBVAC in a Phase 3 efficacy trial in HIV-uninfected infants born to HIV-infected and HIV-uninfected mothers as compared to standard of care BCG vaccination.
The EDCTP (European & Development Countries Clinical Trials Partnership) and Biofabri are supporting this clinical trial. The project is part of the EDCTP2 programme supported by the EU.
Governance
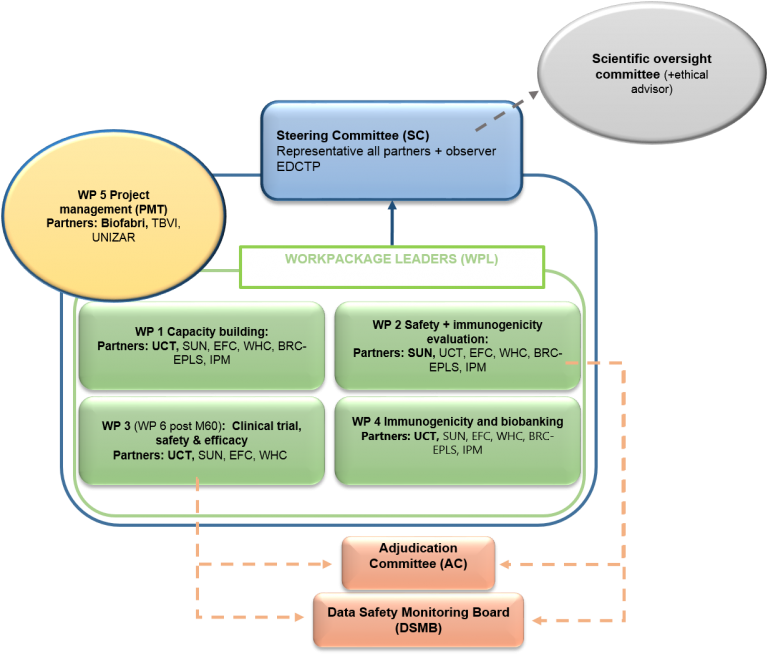
Project Overview
To demonstrate safety, immunogenicity and improved efficacy of the new live attenuated M. tuberculosis vaccine called MTBVAC in a Phase 3 efficacy trial in HUU and HEU newborns, as compared to standard of care BCG vaccination
- Duration: January 2021 – December 2028
- Funded by EDCTP & Biofabri
- Project Management and Clinical Advice by TBVI
- 9 partners
Partners
The MTBVACN3 consortium is composed of nine partners in total, with three European and six sub-Saharan African partners.
- Four participants are Universities and Higher Education Institutions (SATVI/UCT, SUN, Unizar, WHC)
- Three are Research Organizations (IPM, CRB-EPLS, ECF)
- One is a product development partnership supporting and coordinating TB vaccine development (TBVI)
- One is private industry (BIOFABRI)
For this efficacy trial, the Consortium is composed of six recruiting sites. Four in South Africa,, one in Senegal and one in Madagascar.
The project builds upon a group of TB vaccine development partners in Europe and sub-Saharan Africa established in a previous EDCTP-supported project Phase 2a dose-defining study of MTBVAC. It creates an expanded consortium of clinical trial partners for the optimal implementation of a large infant efficacy trial of MTBVAC in high TB incidence settings.
New capacity for efficacy trials in infants will be a valuable resource for the TB vaccine development community. The project creates a network of institutions in three TB endemic African countries with enhanced laboratory capacity to conduct TB vaccine immunology studies and to bio-bank samples to discover immune correlates of vaccine-mediated protection.

TBVI provides its longstanding project management and coordination expertise to support the coordinator (Biofabri) in its role to coordinate and manage the MTBVACN3 project. In addition, TBVI provides its regulatory and clinical expertise to advice on the immunological, clinical and capacity building activities.

University of Zaragoza (Unizar): As the discoverer and initial developer of MTBVAC, Unizar works in close collaboration with industrial partner Biofabri providing longstanding preclinical, immunological and scientific expertise on the molecular bases of attenuation and protection of MTBVAC. As part of the EDCTP MTBVACN3 project, Unizar will provide advice on the proposed immunological, clinical and capacity building activities. Unizar will also provide scientific advice to all partners on the Clinical Trial Protocols and corresponding immunological studies and will contribute to the immunological validation of once-identified TB-diagnostic test suitable for MTBVAC clinical evaluation and the coordination of immunological studies.

Biofabri is the coordinator of this project. It has extensive experience accumulated over decades with live mycobacterial vaccines and is the manufacturer of the MTBVAC vaccine for the trial. Biofabri has successfully conducted the Phase 1b clinical trial, the results of which were published in Lancet Respiratory in August 2019. Biofabri is also conducting the Phase 2a clinical trial of MTBVAC.
FHI Clinical SA provides both operational services such as regulatory, regional project management, clinical monitoring & site management, medical monitoring & pharmacovigilance as well as back-end services such as data management, biostatistics and medical writing to support clinical research activities across the Sub-Saharan region. For on-site monitoring activities, FHI-Clinical SA will deploy its personnel located strategically across Sub-Saharan region.

SATVI/UCT SATVI is a university-accredited TB research centre established in 2001 and located within the University of Cape Town (UCT), South Africa. It is the largest dedicated TB vaccine research group on the African continent. SATVI’s research scope spans several disciplines including paediatrics, infectious diseases, epidemiology, public health, immunology, systems biology and clinical sciences. As partner to the Phase 3 trial, SATVI will utilize its extensive experience to carry out the clinical trial and capacity building activities of the grant.
ECF, The Enhancing Care Foundation, clinical trials unit in eThekwini, South Africa is based at the King Edward VIII Hospital (KEH) and Wentworth hospital (WWH). KEH is a large regional hospital has over 800 beds and sees approximately 22 000 outpatients a month while WWH is a small district level hospital. For this MTBVACN3-trial, ECF will provide all clinical trial infrastructure including physical environment, technical and human resources required to conduct the clinical study in Durban, South Africa
Wits-VIDA With more than 25 years of experience performing clinical trials, including TB vaccine trials, and conveniently situated at the Chris Hani Baragwanath Hospital in Soweto, the Wits Vaccines and Infectious Diseases Analytics Research Unit (Wits-VIDA) has been instrumental in enrolling large numbers of participants in pivotal vaccine trials and generally has a retention rate of >95%. Wits-VIDA will provide their extensive clinical trial experience, infrastructure and expertise to successfully conduct the trial.

CRB-EPLS in Senegal provides their extensive local, clinical and immunological expertise to MTBVAC safety and immunological evaluation in newborns and research of potential biomarkers of vaccine-induced protection.

Publications
- Efficacy, Safety and Immunogenicity Evaluation of MTBVAC in Newborns in Sub-Saharan Africa (MTBVACN3) ClinicalTrials.gov Identifier: NCT04975178
- MTBVAC: A Tuberculosis Vaccine Candidate Advancing Towards Clinical Efficacy Trials in TB Prevention Sergio Lacámara a, Carlos Martin – https://doi.org/10.1016/j.arbres.2023.09.009
News
MTBVACN3 Progress Meeting 2025 held in Baiona, Spain
MTBVACN3 study reports 4,500 newborns vaccinated, moving closer to the first new TB vaccine in a century
The MTBVACN3 consortium gathered in Baiona, Spain, from May 26–28, 2025, for its third annual progress meeting, reviewing advances in the phase III clinical trial of the MTBVAC TB vaccine. The trial, co-funded by the European & Developing Countries Clinical Trials Partnership (EDCTP), is assessing the safety and efficacy of MTBVAC compared with BCG in 7,120 healthy, BCG-naïve newborns.
Launched in 2021 with first enrolments in 2022, the study has already vaccinated around 4,500 participants, keeping the project largely on track to complete randomisation.
Over three days, representatives from all clinical trial sites and other consortium partners shared updates and engaged in detailed discussions on safety monitoring, efficacy assessments, TB investigations, and biostatistics, alongside strategic and operational issues. Sessions also focused amongst others on participant retention, community engagement, and (financial) reporting.
The meeting concluded with a comprehensive overview of the MTBVAC vaccine’s journey from preclinical studies to the current phase III trial—marking a critical step in the effort to develop the first new TB vaccine in nearly a century.
If successful, MTBVAC could replace BCG and offer renewed hope in the fight against tuberculosis, which remains one of the world’s leading infectious killers.
Institut Pasteur Madagascar presents at the TBVI Symposium
At the TBVI Symposium in Les Diablerets (Switzerland), which was held 28-29 January 2025, Institut Pasteur Madagascar showed their strong involvement in the MTBVACN3 study with a video. In this video a young mother from Madagascar inspired many attendees during the TBVI symposium in Les Diablerets by sharing her powerful message about the vital role of participating in a clinical trial in the fight against TB.
You can watch this video here: Young Malagasi mother participates in the MTBVAC study


Annual Progress Meeting 2024
From 27-28 May 2024, the second annual progress meeting of the MTBVAC phase III clinical trial EDCTP co-funded project took place in Johannesburg and Pilanesberg in South Africa. For two days the members of the MTBVACN3 consortium informally and formally discussed in-depth the project progress achieved in the recent 12 months.
Besides presentations in which the partners provided a comprehensive update and overview of the overall progress of the project and insights into the status of the clinical trial, the attendees visited the research and clinical labs and vaccination facilities as well as the pharmacy of Wits-Vida in Johannesburg.
First annual project meeting, 15-18 May 2023
From 15-18 May 2023, the first annual progress meeting of the MTBVAC phase III clinical trial EDCTP co-funded project took place in Andasibe and Antananarivo, Madagascar.
The consortium, composed of nine partners in total of which six recruiting sites (four in South Africa, one in Senegal and one in Madagascar), informally and formally discussed the project progress achieved in the recent 12 months. The attendees visited the Institut Pasteur de Madagascar in Antananarivo with its research and clinical labs and vaccination facilities.
At the meeting venue, a comprehensive update and overview of the overall project progress was presented by all partners and insights into the clinical trial status were discussed. The sites are recruiting at an increasing rate, with almost 300 newborns by May, 2023.

MTBVACN3 will evaluate safety and efficacy of MTBVAC administered intradermally in 7120 healthy, BCG-naïve, HIV-uninfected newborns born to HIV-uninfected mothers and HIV-infected mothers without known exposure to close/household TB contacts.
All in all, the days hosted by the IPM-team, provided a platform to share extensively experiences, challenges and solutions and to look forward to the next steps in the project.
First Site Initiation Visit
23 September the first Site Initiation Visit (SIV) of the clinical trial “MTBVAC in Newborns: Randomised, Double-blind Controlled Phase 3 Trial to Evaluate the Efficacy, Safety, and Immunogenicity of MTBVAC Administered in Healthy HIV Unexposed and HIV Exposed Uninfected Newborns in Tuberculosis-endemic Regions of Sub-Saharan Africa” was performed. This first visit took place at the South African Tuberculosis Vaccine Initiative – University of Cape Town (SATVI-UCT), one of the six different clinical sites across Africa.
Kick off Meeting MTBVAC Phase III
From 10-12 May 2022, the Kick-off Meeting (KoM) of the MTBVAC phase III clinical trial EDCTP co-funded project was held in Baiona, Spain. With the only licensed TB vaccine, Bacillus Calmette-Guérin (BCG), having limited impact on prevention of TB disease and transmission of Mycobacterium tuberculosis, there is an urgent need for a new, improved TB vaccine. MTBVAC is a live attenuated M. tuberculosis-based vaccine with two major virulence factors removed, which is currently entering a randomised, double-blinded, active BCG-controlled phase III clinical trial. This study will evaluate the safety and efficacy of MTBVAC administered by intradermal route in 7120 healthy, BCG-naïve, HIV-uninfected newborns born to HIV-uninfected mothers and HIV-infected mothers without known exposure to close/household TB contacts.
For three days, members of the MTBVACN3 consortium had the opportunity to get to know each other and discuss the details of this study. During the first session, the design of this project was outlined, including an update on the work done so far and a description of the trial endpoints and safety considerations. This general overview provided the frame to further discuss the protocol parameters on the second day, including the statistical design, procedures, timelines, and logistics involving vaccine distribution and sample management. In two dedicated break-out sessions the participants therefore put together and discussed the most urgent and important risk issues and a management plan and shared their expertise with community engagement. Both sessions strongly underlined the importance of efficient exchange of knowledge and being optimally prepared well in advance to ensure successful performance and completion of this study.
The team is excited to enter this important next phase!

Work packages
Work packages
WP1 Capacity building and training in Quantiferon and whole blood assays
WP2 Safety and Immunogenicity studies
WP3 Pivotal Safety & Efficacy until first interim analysis
WP4 Immunogenicity evaluation of samples from WP2, WP3
This work package will be led by University of Cape Town (UCT) and is dedicated to conduct the immunogenicity evaluation of the processed samples taken in work packages 1 and 2.
WP5 Project management
Next steps following the MTBVACN3 project
Acknowledgement

Acknowledgement of EDCTP in publications, presentations, posters:
