
Words from the chairs
“TBVAC-HORIZON provides a unique environment of multidisciplinary research within a Consortium of leading European institutions in the area of academic, clinical and commercial tuberculosis R&D. Its research spans basic and translational science with an interdisciplinary and integrated approach, including clinical studies. This work will advance our understanding of protective immunity, particularly at the site of infection, the lung, consequently facilitating the development of novel TB vaccine candidates. TBVAC-HORIZON will thus innovate and diversify the existing TB vaccine pipeline. As with previous TBVI-led consortia, partners work together to deliver excellent scientific results and outcomes that will secure Europe’s leading role in the development of more effective TB vaccines.”


Prof. Helen McShane, Chair TBVAC-HORIZON’s PI Translational Research
Prof. Steffen Stenger, Chair TBVAC-HORIZON’s PI Basic Research
Project overview
TBVAC-HORIZON: Innovating and diversifying the TB vaccine pipeline
- Duration: April 2023 – March 2027
- Funded by the European Union under HaDEA; type of action HORIZON-RIA
- Coordinated by TBVI
- 19 partners
Aim and objectives
While there are few vaccine candidates in late-stage clinical trials, the TB vaccine pipeline remains insufficient and needs diversification and innovation. To ensure that the most effective and affordable vaccines are developed, innovation by new platforms and strategies is needed.
TBVAC-HORIZON will address this through in-depth investigation of the mechanisms of immune responses to infection in the lung, which will identify biomarkers to rationalise vaccine design and improve monitoring of vaccine immunity.
The translational component of the project includes head-to-head comparison of novel candidate vaccines in standardised animal models, aligned comparative experimental medicine studies in humans and non-human primates, and assessment of immune responses in individuals with comorbidity-induced increased susceptibility to TB. It will also establish novel delivery systems and adjuvant formulations. Finally, a novel GMP platform for live attenuated vaccines will be developed.
The combination of basic, applied and translational research, preclinical efficacy and human experimental medicine studies, improved adjuvants, and novel GMP platforms will pave the way for novel concepts for candidate vaccines and improved immunisation strategies with the ultimate goal of accelerated availability of affordable, accessible and more effective TB vaccines.
Work packages
TBVAC-HORIZON is divided into five work packages:
- WP 1: Mechanisms of immune protection in the M. tb-infected lung
- WP 2: Improving lung immunity by mucosal vaccine delivery (Translational Vaccine Research)
- WP 3: Identifying biomarker assays indicating vaccine-induced protection
- WP 4: Innovating expression, formulation, evaluation and GMP tools for next generation TB vaccine development
- WP 5: Project coordination
Project governance:
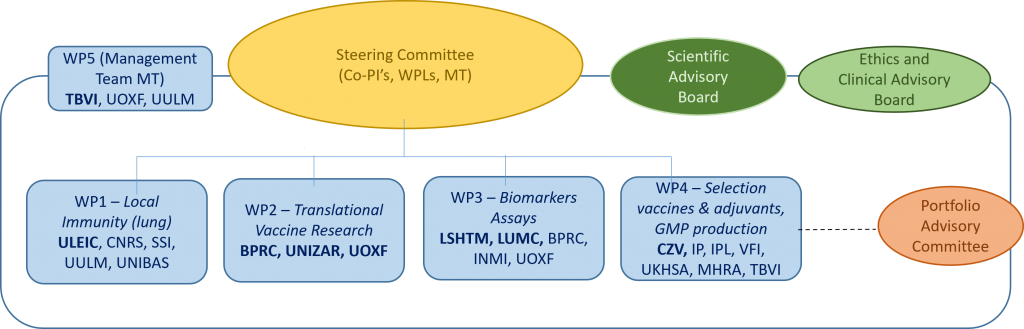
The consortium will be advised by the Scientific Advisory Board, the Ethics and Clinical Advisory, and the Portfolio Advisory Committee.
Partners:

TBVAC-HORIZON consortium
The TBVAC-HORIZON consortium consists of 19 partners and builds upon over two decades of collaborative R&D and has been the major contributor to the current global TB vaccine pipeline.
- Istituto Nazionale per le Malattie Infettive ‘Lazzaro Spallanzani’ (INMI-IRCCS) – PI Delia Goletti
- Institut Pasteur (IP) – PI Roland Brosch
- Institut Pasteur de Lille (IPL) – PI Camille Locht
- Leiden University Medical Centre (LUMC) – PI Simone Joosten
- Statens Serum Institut (SSI) – PI Rasmus Mortensen
- University of Zaragoza (UNIZAR) – PI Nacho Aguilo
- University of Ulm (UULM)
- CZ Vaccines, S.A. (CZV) – PI Dolores Montenegro
- University of Basel (UNIBAS) – PI Daniel Pinschewer & Carolyn King
- Vaccine Formulation Institute CH, Ltd. (VFI)
- Department of Health (UKHSA) – PI Simon Clark
- London School of Hygiene and Tropical Medicine (LSHTM) – PI Hazel Dockrell & Jayne Sutherland
- Medicines and Healthcare Products Regulatory Agency (MHRA) – PI Phil Hogarth
- University of Leicester (ULEIC) – PI Andrea Cooper
- University of Oxford (UOXF) – PI Helen McShane
Advisory Boards
The Scientific Advisory Board, the Ethics and Clinical Advisory, and the Portfolio Advisory Committee will advise the TBVAC-HORIZON consortium.
Members of the Scientific Advisory Board:
 Ann M. Ginsberg, M.D., Ph.D., former Deputy Director, TB Vaccines in the Global Health Division of the Gates Foundation. She has conducted and managed TB research and product development programs for 25 years, including 15 years leading and designing clinical strategy for development of TB vaccines and drug regimens for the developing world. Ann previously served as the Chief, Respiratory Diseases Branch, NIAID, NIH, as Director, Project Management at Merck Research Laboratories, as Chief Medical Officer at the Global Alliance for TB Drug Development, as Chief Medical Officer at Aeras and as Senior Technical Advisor at IAVI. In these roles, she oversaw the early clinical development program of Pretomanid and two recent, positive TB vaccine efficacy trials – of BCG revaccination and M72/AS01E. Ann has served on numerous national and international advisory committees on vaccines and global health, including the U.S. National Vaccine Advisory Committee. Her AB cum laude in Biology is from Harvard University, her PhD in Molecular Biology from Washington University and her MD from Columbia University. She was Board-certified in Anatomic Pathology.
Ann M. Ginsberg, M.D., Ph.D., former Deputy Director, TB Vaccines in the Global Health Division of the Gates Foundation. She has conducted and managed TB research and product development programs for 25 years, including 15 years leading and designing clinical strategy for development of TB vaccines and drug regimens for the developing world. Ann previously served as the Chief, Respiratory Diseases Branch, NIAID, NIH, as Director, Project Management at Merck Research Laboratories, as Chief Medical Officer at the Global Alliance for TB Drug Development, as Chief Medical Officer at Aeras and as Senior Technical Advisor at IAVI. In these roles, she oversaw the early clinical development program of Pretomanid and two recent, positive TB vaccine efficacy trials – of BCG revaccination and M72/AS01E. Ann has served on numerous national and international advisory committees on vaccines and global health, including the U.S. National Vaccine Advisory Committee. Her AB cum laude in Biology is from Harvard University, her PhD in Molecular Biology from Washington University and her MD from Columbia University. She was Board-certified in Anatomic Pathology.
 Dr. Sam Behar is Professor of Microbiology and Physiological Systems at the University of Massachusetts Chan Medical School, where he studies immunity to Mycobacterium tuberculosis. Dr. Behar and the members of his lab have made important contributions to our understanding of immunity to tuberculosis including T cell priming, acquisition of effector function, and memory responses. By understanding mechanisms of host resistance to tuberculosis, including how T cell responses are coordinated and integrated in vivo, and how bacteria evade immunity, his ultimate goal is to inform vaccine design and testing.
Dr. Sam Behar is Professor of Microbiology and Physiological Systems at the University of Massachusetts Chan Medical School, where he studies immunity to Mycobacterium tuberculosis. Dr. Behar and the members of his lab have made important contributions to our understanding of immunity to tuberculosis including T cell priming, acquisition of effector function, and memory responses. By understanding mechanisms of host resistance to tuberculosis, including how T cell responses are coordinated and integrated in vivo, and how bacteria evade immunity, his ultimate goal is to inform vaccine design and testing.
 Dr Ann Rawkins nee Williams a semi-retired Scientific Leader whose previous role at Public Health England (now UK Health Security Agency) Porton Down, Salisbury UK was to provide scientific direction to the TB research programme which aimed to identify and develop vaccine and/or therapeutic targets and evaluate these in aerosol-challenge in-vivo models. She established and led a vaccine evaluation team which became a key component of the European effort to develop an improved TB vaccine and which continues under the leadership of Dr Simon Clark. She has been a work package leader in several EU-funded TB vaccine consortia and was a Community lead in the BMGF Collaboration for TB Vaccine Development. Prior to her TB studies, she worked at the same site in the Centre for Applied Microbiology and Research studying Salmonella infection in chickens for 4 years and before that conducted her PhD studies on the pathogenic mechanisms of Legionnaires’ disease.
Dr Ann Rawkins nee Williams a semi-retired Scientific Leader whose previous role at Public Health England (now UK Health Security Agency) Porton Down, Salisbury UK was to provide scientific direction to the TB research programme which aimed to identify and develop vaccine and/or therapeutic targets and evaluate these in aerosol-challenge in-vivo models. She established and led a vaccine evaluation team which became a key component of the European effort to develop an improved TB vaccine and which continues under the leadership of Dr Simon Clark. She has been a work package leader in several EU-funded TB vaccine consortia and was a Community lead in the BMGF Collaboration for TB Vaccine Development. Prior to her TB studies, she worked at the same site in the Centre for Applied Microbiology and Research studying Salmonella infection in chickens for 4 years and before that conducted her PhD studies on the pathogenic mechanisms of Legionnaires’ disease.
Members of the Ethics and Clinical Advisory:
 Michèle Tameris, MBChB, is a South African who qualified as a medical doctor in 1980 at the University of Cape Town, followed by 20 years practicing in the state health services. In 2003 she joined the staff of South African Tuberculosis Vaccine Initiative (SATVI) within the University of Cape Town as a clinical researcher based at their field site in Worcester where she has been actively involved as principal or sub-investigator in 32 TB vaccine research trials of 10 novel candidates, as well as TB treatment and diagnostic trials. In 2022 she received ad hominem promotion to Chief Research Officer with the title of Associate Professor.
Michèle Tameris, MBChB, is a South African who qualified as a medical doctor in 1980 at the University of Cape Town, followed by 20 years practicing in the state health services. In 2003 she joined the staff of South African Tuberculosis Vaccine Initiative (SATVI) within the University of Cape Town as a clinical researcher based at their field site in Worcester where she has been actively involved as principal or sub-investigator in 32 TB vaccine research trials of 10 novel candidates, as well as TB treatment and diagnostic trials. In 2022 she received ad hominem promotion to Chief Research Officer with the title of Associate Professor.
She received Wellcome Trust International Engagement Awards in 2012 and 2014 for two drama projects targeting respectively adolescents and the community at large. She is a core member of the Stop TB Working Group for new Vaccines.
 Dr Norbert Stockhofe is qualified as veterinarian and working as senior veterinary pathologist at Wageningen Bioveterinary Research, part of Wageningen University & Research. Since over 30 years he is working on infectious diseases in farm animals and humans using target animal and biomedical animal models. He has participated in more than 10 EU projects on vaccine and infectious disease research. Since more than 25 years he is participating in animal experimentation comities and for nearly 10 years head of one Animal Welfare Body of Wageningen University & Research.
Dr Norbert Stockhofe is qualified as veterinarian and working as senior veterinary pathologist at Wageningen Bioveterinary Research, part of Wageningen University & Research. Since over 30 years he is working on infectious diseases in farm animals and humans using target animal and biomedical animal models. He has participated in more than 10 EU projects on vaccine and infectious disease research. Since more than 25 years he is participating in animal experimentation comities and for nearly 10 years head of one Animal Welfare Body of Wageningen University & Research.
Members of the Portfolio Advisory Committee (PAC):
 Dr Elly van Riet is a senior scientist at TBVI, using her 15 years of experience in several aspects of vaccine research, to move the development of safe, effective and affordable TB vaccines forward. She earned her MSc as an engineer in biotechnology at Wageningen University and Research Centre, followed by a Ph.D. in immunology at the Department of Parasitology, Leiden University Medical Centre, both in The Netherlands. She has worked on vaccine development ever since, first as a postdoc at the Leiden Academic Centre for Drug Research (The Netherlands). She was granted a postdoctoral fellowship by the ‘Japan Society for the Promotion of Science’ at Research Center of the National Institute of Infectious Diseases (Tokyo, Japan). In 2013 she joined Intravacc, a Dutch organization developing vaccines from discovery to phaseI/II clinical trials, as head of the department Clinical Development and later as Program Manager of Innovation, Program Manager of Bacterial Vaccines and Vice President R&D.
Dr Elly van Riet is a senior scientist at TBVI, using her 15 years of experience in several aspects of vaccine research, to move the development of safe, effective and affordable TB vaccines forward. She earned her MSc as an engineer in biotechnology at Wageningen University and Research Centre, followed by a Ph.D. in immunology at the Department of Parasitology, Leiden University Medical Centre, both in The Netherlands. She has worked on vaccine development ever since, first as a postdoc at the Leiden Academic Centre for Drug Research (The Netherlands). She was granted a postdoctoral fellowship by the ‘Japan Society for the Promotion of Science’ at Research Center of the National Institute of Infectious Diseases (Tokyo, Japan). In 2013 she joined Intravacc, a Dutch organization developing vaccines from discovery to phaseI/II clinical trials, as head of the department Clinical Development and later as Program Manager of Innovation, Program Manager of Bacterial Vaccines and Vice President R&D.
 Dr Barry Walker is former Vice President of Preclinical Development (Aeras, USA), and previously, Principal Scientist at the National Institute for Biological Standards and Control, NIBSC, UK. He has over 30 years’ experience in vaccine discovery and translational development of biologicals, with 20 years’ experience in a regulatory environment. As Vice President Preclinical Development at Aeras he focussed and implemented the strategy for the clinical immune studies and vaccine development programme for Aeras for over 4 years and brought 4 new vaccine strategies from concept to clinical development. Prior to this he was Principal Scientist and PI at the Immunology and Cellular Immunity Section and the Bacteriology Division at NIBSC. In these roles he has also advised on strategic implementation of specific programmes funded by the EC, BMGF and the NIH. Currently acting as an independent scientific consultant translational development of biologicals with an honorary position at University of Surrey.
Dr Barry Walker is former Vice President of Preclinical Development (Aeras, USA), and previously, Principal Scientist at the National Institute for Biological Standards and Control, NIBSC, UK. He has over 30 years’ experience in vaccine discovery and translational development of biologicals, with 20 years’ experience in a regulatory environment. As Vice President Preclinical Development at Aeras he focussed and implemented the strategy for the clinical immune studies and vaccine development programme for Aeras for over 4 years and brought 4 new vaccine strategies from concept to clinical development. Prior to this he was Principal Scientist and PI at the Immunology and Cellular Immunity Section and the Bacteriology Division at NIBSC. In these roles he has also advised on strategic implementation of specific programmes funded by the EC, BMGF and the NIH. Currently acting as an independent scientific consultant translational development of biologicals with an honorary position at University of Surrey.
 Prof. Dr Gideon Kersten is a vaccinologist with over 35 years of experience in vaccine development. His special interests are in characterization and formulation of vaccines and other biologicals. At Institute for Public Health in the Netherlands (RIVM) he was involved in the development of new vaccines and improving existing ones. These included vaccines against polio, influenza, RS virus, Neisseria meningitidis type B and pertussis. He was responsible for formulation development and vaccine characterization. Since 2012 he holds a special professorship in vaccine development at the Leiden Academic Center for Drug Research . In 2017 he became Chief Scientific Officer of Intravacc. In 2020 he joined Coriolis Pharma, a contract research organization located near Munich and dedicated to the characterization and formulation of biologics. At Coriolis he is a scientific reviewer and scientific advisor.
Prof. Dr Gideon Kersten is a vaccinologist with over 35 years of experience in vaccine development. His special interests are in characterization and formulation of vaccines and other biologicals. At Institute for Public Health in the Netherlands (RIVM) he was involved in the development of new vaccines and improving existing ones. These included vaccines against polio, influenza, RS virus, Neisseria meningitidis type B and pertussis. He was responsible for formulation development and vaccine characterization. Since 2012 he holds a special professorship in vaccine development at the Leiden Academic Center for Drug Research . In 2017 he became Chief Scientific Officer of Intravacc. In 2020 he joined Coriolis Pharma, a contract research organization located near Munich and dedicated to the characterization and formulation of biologics. At Coriolis he is a scientific reviewer and scientific advisor.
 Dr Simon Clark is Principal Scientist in the Pre-clinical team of the Vaccine Development Evaluation Centre at the UK Health Security Agency, Porton Down, UK.
Dr Simon Clark is Principal Scientist in the Pre-clinical team of the Vaccine Development Evaluation Centre at the UK Health Security Agency, Porton Down, UK.
Dr. Simon Clark provides scientific direction to the TB research programme which aims to develop and utilise aerosol-challenge in-vivo models to identify vaccine and/or therapeutic candidates. Dr. Clark has over 20 years of experience in the pre-clinical evaluation of interventions against human diseases (including TB & NTM infections). Dr. Clark has established and refined aerosol generation and delivery systems allowing characterisation of pathogens in aerosols, and infection. He leads in vivo microbiologists, immunologists and aerobiologists and has collaborated globally with the majority of the key developers.
 Dr Philip Hogarth, Medical and Health products Regulatory Agency (MHRA), is an immunologist, gaining his PhD at the Liverpool School of Tropical Medicine in 1997. After postdoc positions at Bristol University, he joined APHA in 2001 to recruit/lead a group developing mouse models of bovine TB: screening novel vaccines, vaccine regimens and administration routes to inform cattle infection models. A particular area of focus was mechanisms and correlates of immunity induced by BCG and BCG prime-boost approaches. Using high parameter flow cytometry, Phil’s team pioneered UK work on the role of lung CD4 T cell tissue-resident memory cells to BCG. The group defined and translated relevant immune parameters/assays from murine to cattle vaccination/infection models, developing novel reagents and assays where required. In 2018, Phil moved into a science:policy interface role at Defra as TB science Subject Matter Expert, developing R&D strategies in response to the 2018 Godfray Review on behalf of England and the Devolved Administrations on Scotland & Wales. In 2020 he returned to APHA as TB Lead Scientist, leading the TB Portfolio in R&D, Surveillance, Field and Data Sciences. During his tenure, Phil introduced significant advancements to statutory bTB confirmation and surveillance, implementing molecular disease confirmation, and WGS/geographic-linked phylogenetic investigation for frontline outbreak characterisation He also transitioned CattleBCG and the BCG compatible DIVA test from research projects into regulatory field trials in preparation for Marketing Authorisations and rollout in England & Wales. In September 2023, Phil accepted a new role, as Head of Vaccines R&D at the MHRA, where he leads a diverse division with 7 research groups which includes TB.
Dr Philip Hogarth, Medical and Health products Regulatory Agency (MHRA), is an immunologist, gaining his PhD at the Liverpool School of Tropical Medicine in 1997. After postdoc positions at Bristol University, he joined APHA in 2001 to recruit/lead a group developing mouse models of bovine TB: screening novel vaccines, vaccine regimens and administration routes to inform cattle infection models. A particular area of focus was mechanisms and correlates of immunity induced by BCG and BCG prime-boost approaches. Using high parameter flow cytometry, Phil’s team pioneered UK work on the role of lung CD4 T cell tissue-resident memory cells to BCG. The group defined and translated relevant immune parameters/assays from murine to cattle vaccination/infection models, developing novel reagents and assays where required. In 2018, Phil moved into a science:policy interface role at Defra as TB science Subject Matter Expert, developing R&D strategies in response to the 2018 Godfray Review on behalf of England and the Devolved Administrations on Scotland & Wales. In 2020 he returned to APHA as TB Lead Scientist, leading the TB Portfolio in R&D, Surveillance, Field and Data Sciences. During his tenure, Phil introduced significant advancements to statutory bTB confirmation and surveillance, implementing molecular disease confirmation, and WGS/geographic-linked phylogenetic investigation for frontline outbreak characterisation He also transitioned CattleBCG and the BCG compatible DIVA test from research projects into regulatory field trials in preparation for Marketing Authorisations and rollout in England & Wales. In September 2023, Phil accepted a new role, as Head of Vaccines R&D at the MHRA, where he leads a diverse division with 7 research groups which includes TB.

TBVAC-HORIZON receives funding from the European Union’s Horizon Europe research and innovation programme under grant agreement No 101080309. It is funded by the European Union, the UK Research and Innovation, and the Swiss State Secretariat for Education, Research and Innovation. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or European Health and Digital Executive Agency (HADEA). Neither the European Union, UKRI, SERI nor the granting authority can be held responsible for them.
Publications
Comparative study of [18F]DPA714 and [18F]FDG PET tracers in an Experimental Model of Pulmonary Tuberculosis
Stammes M, Vierboom M, Sombroek C, Bakker J, Meijer L, Vervenne R, Hofman S, Nutma E, Kondova I, Windhorst A, Langermans J, and Verreck F
Molecular Imaging and Biology 2025, 10.1007/s11307-025-02057-6
EPublished: 21 October 2025
Improved Immune Responses and Tuberculosis Protection by Aerosol Vaccination with recombinant BCG expressing ESX-1 from Mycobacterium marinum
Sayes F, Frigui W, Pawlik A, Tillier C, Tichit M, Hardy D, Brosch R
BioRxiv 10.1101/2025.09.18.677039
Preprint: 18 September 2025
Characterization of QuantiFERON-TB-Plus Results in Patients with Tuberculosis Infection and Multiple Sclerosis
Petruccioli E, Prosperini L, Ruggieri S, Vanini V, Salmi A, Cuzzi G, Galgani S, Haggiag S, Tortorella C, Parisi G, D’Agostino A, Gualano G, Palmieri F, Gasperini C, Goletti D
Neurol Int. 2025, 10.3390/neurolint17080119. PMID: 40863988
Published: 2 August 2025
Immune dysregulation of diabetes in tuberculosis
Thong PM, Wong YH, Kornfeld H, Goletti D, Ong CWM
Semin Immunol. 2025 Jun;78:101959, doi: 10.1016/j.smim.2025.101959, PMID: 40267700
EPublished: 22 April 2025
World TB Day 2025 Theme “Yes! We Can End TB: Commit, Invest, Deliver” can be made a reality through concerted global efforts to advance diagnosis, treatment and research of tuberculosis infection and disease
Goletti D, Matteelli A, Cliff JM, Meintjes G, Graham S, Esmail H, Shan Lee S.
Int J Infect Dis. 2025 Jun;155:107892. doi: 10.1016/j.ijid.2025.107892. PMID: 40107343
EPublished: 17 March 2025
Diagnostic tests for tuberculosis infection and predictive indicators of disease progression: Utilizing host and pathogen biomarkers to enhance the tuberculosis elimination strategies
Alonzi T, Petruccioli E, Aiello A, Repele F, Goletti D.
. Int J Infect Dis. 2025 Jun;155:107880. doi: 10.1016/j.ijid.2025.107880, PMID: 40086617
EPublished: 12 March 2025
Therapy modulates the response to T cell epitopes over the spectrum of tuberculosis infection
Petrone L, Peruzzu D, Altera AMG, Salmi A, Vanini V, Cuzzi G, Coppola A, Mellini V, Gualano G, Palmieri F, Panda S, Peters B, Sette A, Lindestam Arlehamn C, Goletti D
Journal of Infection, 10.1016/j.jinf.2024.106295
Published: December 2024
Altered hepatic metabolic landscape and insulin sensitivity in response to pulmonary tuberculosis
Das M K, Savidge B, Pearl J E, Yates T, Miles G, Pareek M, Haldar P, Cooper A M
PLoS Pathogens, 10.1371/journal.ppat.1012565
Published: 27 September 2024
Detection of Mycobacterium tuberculosis DNA in CD34+ peripheral blood mononuclear cells of adults with tuberculosis infection and disease
Repele F, Alonzi T, Navarra A, Farroni C, Salmi A, Cuzzi G, Delogu G, Gualano G, Puro V, De Carli G, Girardi E, Palmieri F, Martineau A R, Goletti D
International Journal of Infectious Diseases, 10.1016/j.ijid.2024.106999
Published: April 2024
Drugs for treating infections caused by non-tubercular mycobacteria: a narrative review from the study group on mycobacteria of the Italian Society of Infectious Diseases and Tropical Medicine
Calcagno A, Coppola N, Sarmati L, Tadolini M, Parella R, Mateelli A, Riccardi N, Trezzi M, Di Biagio A, Pirriatore V, Russo A, Gualano G, Pontali E, Surace L, Falbo E, Mencarini J, Palmieri A, Gori A, Schiuma M, Lapadula G, Goletti D
Infection, 10.1007/s15010-024-02183-3
Published: 8 February 2024
Impact of aging on immunity in the context of COVID-19, HIV, and tuberculosis
Grifoni A, Alonzi T, Alter G, McClain Noonan D, Landay A L, Albini A, Goletti D
Frontiers in Immunology, 10.3389/fimmu.2023.1146704
Published: 24 May 2023
News







