March 24, 2025
Today, March 24, marks World Tuberculosis Day on the anniversary of Dr. Robert Koch’s groundbreaking discovery of Mycobacterium tuberculosis in 1882. Despite significant global efforts that have saved approximately 79 million lives since 2000, TB remains a major health challenge. In 2023 alone, over 10 million new cases were reported and the disease claimed 1.25 million lives – making it one of the world’s deadliest infectious diseases.
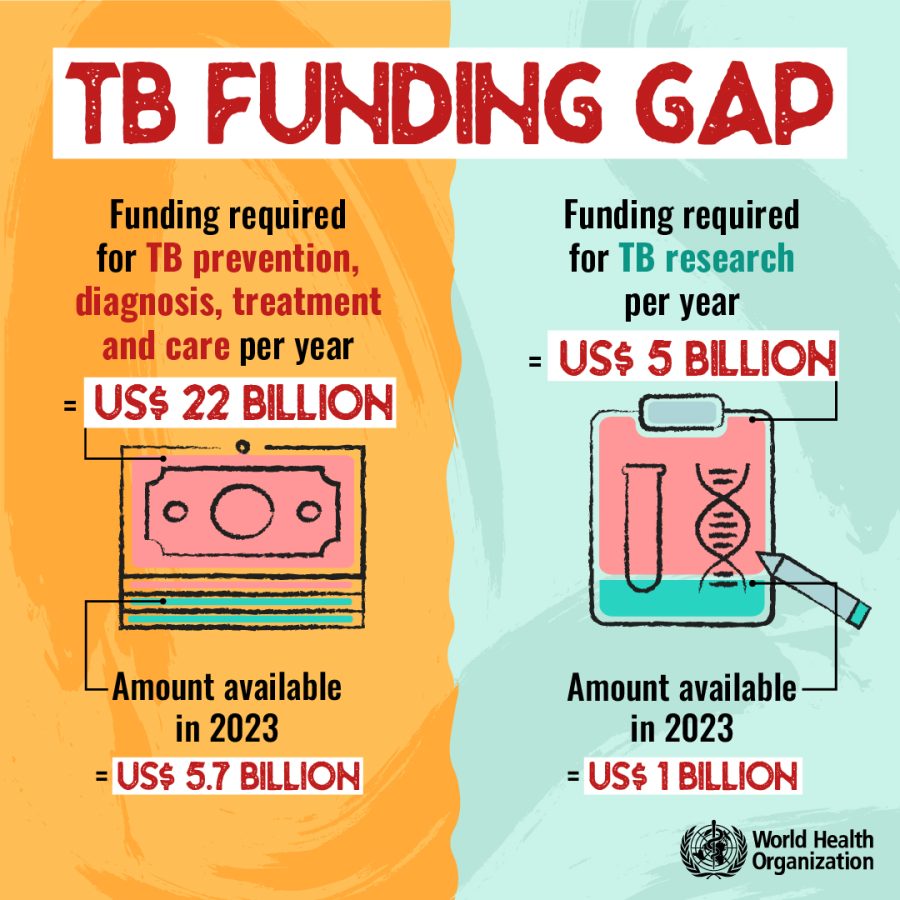
The need for stronger commitment and investment
The European Vaccine Initiative (EVI) and Tuberculosis Vaccine Initiative (TBVI) call for renewed urgency in combating TB. TB predominantly impacts low- and middle-income countries, where challenges such as malnutrition, type 2 diabetes, and HIV co-infection intensify its adverse effects. The development of new and improved vaccines is considered the most cost-effective strategy to meet the WHO’s End TB goals, which target a 95% reduction in TB deaths by 2035 compared to 2015 levels. However, despite several promising vaccine candidates reaching late-stage clinical trials, progress and innovations have been significantly hindered by limited funding.
Collaboration: a key to success
Ending TB requires a united effort by researchers, developers, policymakers, and funders. Through our world-wide network of scientific and business partners, TBVI accelerates TB vaccine research & development and empowers our partners in disease-endemic countries. Strengthening this collaborative network will streamline research, accelerate clinical trials, and ensure equitable vaccine access, particularly in vulnerable communities.
Within a similar collaborative framework, EVI is advancing efforts to combat antimicrobial resistance (AMR), for which TB is one of the major drivers of AMR-associated morbidity and mortality globally. Beyond vaccine development, EVI leads initiatives like the DRAIGON project (www.draigon.eu), which is pioneering an innovative in vitro diagnostic device that integrates whole-genome sequencing and artificial intelligence. This cutting-edge technology enables rapid, near-patient diagnosis of multi-drug resistant infections, further strengthening global efforts to tackle AMR and improve public health outcomes.
.
The TB vaccine development pathway
One of the outcomes of TBVI’s collaborative approach is the establishment of the ‘TB Vaccine Development Pathway’, a strong and innovative web-based tool offering structured guidance on vaccine development, from initial discovery to global implementation. The Pathway was developed in 2018, and is a living document that is regularly updated, always in collaboration with a team of scientific and technical experts and with the input from the global TB vaccine community.

Also currently, an update is prepared to include the latest advances in the field. In a related effort to enhance decision-making in vaccine development and deployment, EVI coordinates the PrIMAVeRa consortium (www.primavera-amr.eu), which is developing an open-source, web-based platform that integrates mathematical models with comprehensive epidemiological data. This tool aims to support policymakers in making data-driven decisions on the prioritisation of vaccines and monoclonal antibodies, ultimately optimising resource allocation in the fight against infectious diseases. PrIMAVeRa is part of the broader AMR Accelerator programme, which includes initiatives like COMBINE, dedicated to strengthening the scientific foundation for AMR and TB research and fostering collaboration across the field.
Investing in future generations
Beyond facilitating vaccine development, TBVI and EVI are also committed to fostering the next generation of researchers. Through our yearly TBVI Awards, we support young scientists to showcase their work and become part of the TB community.
In line with this commitment to capacity building, EVI provides training to junior to mid-career researchers from low- and middle-income countries (LMICs) to acquire specialist skills in clinical research and development through the EDCTP/TDR Clinical Research and Development Fellowship (CRDF) scheme. By strengthening expertise in LMICs, these initiatives contribute to a more sustainable and globally inclusive research ecosystem.
Additionally, advocacy and community- and stakeholder engagement remain crucial components of the fight against TB, ensuring that awareness, policy support, and resource allocation continue to grow.
A call to action
The fight against TB is still ongoing. With strengthened commitment, increased investment, and effective collaboration, we can move closer to eradicating this devastating disease. Together with our partners, we are proud to contribute to the greater goal to End TB.

About EVI
The European Vaccine Initiative (EVI) is a leading European non-profit Product Development Partnership (PDP) that is supporting global efforts to develop effective and affordable vaccines against diseases of poverty and emerging infectious diseases.
www.euvaccine.eu

About TBVI
The TuBerculosis Vaccine Initiative (TBVI) is a non-profit foundation that facilitates the discovery and development of new, safe and effective tuberculosis (TB) vaccines that are accessible and affordable for all people. TBVI brings together partners from around the world by creating an enabling environment for TB vaccine research & innovation and product development, with the ultimate goal to relieve the burden of TB.
www.tbvi.eu

Funding Acknowledgements / Disclaimers:
DRAIGON is Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them.
PrIMAVeRa has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 101034420 (PrIMAVeRa). This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. This communication reflects the author’s view, and neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained herein.


