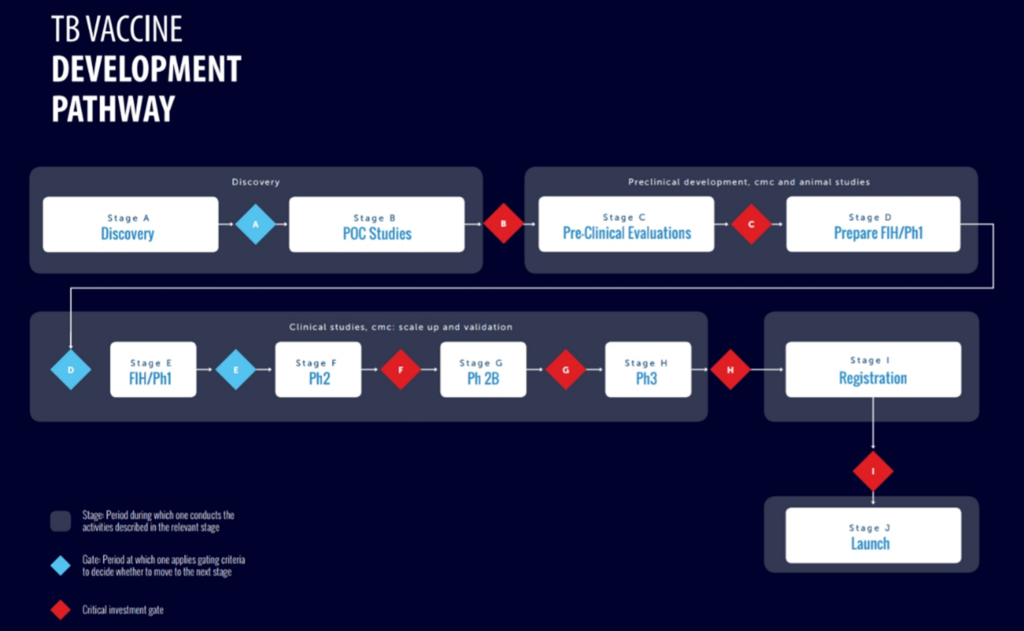
On 12 February 2026, an open online consultation meeting on the TB Vaccine Development Pathway brought together experts and stakeholders to review its recent renewal and gather input for further improvements. This consultation supports coordinated global efforts to accelerate TB vaccine development and improve future access and implementation of TB vaccines for all.
TB Vaccine Development Pathway
Developing a TB vaccine – from an initial idea to public broad availability – is a complex and resource-intensive process. The TB Vaccine Development Pathway addresses the early trajectory of this journey, guiding developers from discovery through product launch and is regularly updated to include the latest scientific insights. Two dedicated expert working groups contributed to the latest revision of the TB Vaccine Development Pathway:
- The animal models working group updated preclinical functions including safety, immunogenicity, and preclinical protection and efficacy.
- The clinical trial design working group revised clinical functions related to development and operations, safety, immunology, and clinical protection and efficacy.
Feedback
Participants were invited to provide feedback to further improve the TB Vaccine Development Pathway. Processing these contributions is currently ongoing. However, as vaccine research continues to evolve, the TB Vaccine Development Pathway will remain a living resource. Stakeholders are invited to share input at any time through the contact form on the website.
Support
To support product development efforts, consulting services are available through a grant from the Gates Foundation, enabling IAVI and TBVI to provide guidance to developers seeking assistance. Information about this service can be found here on our website
The consultation meeting reflects continued international collaboration to accelerate TB vaccine development and advance the shared goal of reducing the burden of TB worldwide.